In this research, Polycaprolactone-graphene oxide Nanocomposite coating was synthesized and characterized by Electrospinning on Ti6AL4V alloy. In order to create a uniform coating with optimal thickness, the effective parameters of Electrospinning coating, including solvent, polymer concentration, and bioceramic percentage, were investigated. Also, the cytotoxicity and corrosion tests were evaluated by the electrochemical polarization test method of the created coating in comparisons and different percentages. In order to characterize the coating, a test such as a scanning electron microscope was used. The results showed that as much as the amount of Graphene oxide is increased, the diameter of Nanofibers decreases. The diameter of Polycaprolactone Nanofibers was 1.3 micrometers, which increases to 56.0 micrometers by adding Graphene oxide. The results of the corrosion test showed that the use of Nano composite coating increased the corrosion resistance to the size of the coating. The nanocomposite coating consists of polycaprolactone nanofibers and graphene oxide, which mimics the behavior of the extracellular matrix and improves the biological and antibacterial behavior of the titanium surface. So far, there has been no report on the creation of this fibrous nanocomposite coating on titanium. The results of the cytotoxicity test showed that the use of Nanocomposite coating has effectively reduced the cytotoxicity on the scaffolds. By creating a polycaprolactone-graphene oxide nanofiber composite coating, the biological and antibacterial properties of titanium alloy will be improved and its corrosion resistance will probably change. In this project, the main question is extracting effective parameters in creating a composite coating on titanium surface by electrospinning method and characterizing and biological evaluation of the created coating.
| Published in | International Journal of Materials Science and Applications (Volume 13, Issue 2) |
| DOI | 10.11648/j.ijmsa.20241302.11 |
| Page(s) | 13-23 |
| Creative Commons |
This is an Open Access article, distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium or format, provided the original work is properly cited. |
| Copyright |
Copyright © The Author(s), 2024. Published by Science Publishing Group |
Polycaprolactone, Graphene Oxide, MTT Assay, Biomaterial, Electrospinning
name of the material | Molecular Weight | chemical formula |
|---|---|---|
Polycaprolactone | 23/480 | C6H10O2 |
ROW | Name of the compound | the amount of |
|---|---|---|
1 | Ethanole | 0/2 mm |
2 | PCL | 0.3 g |
3 | GO | 0.02 g |
4 | chloroform | 1.8mm |
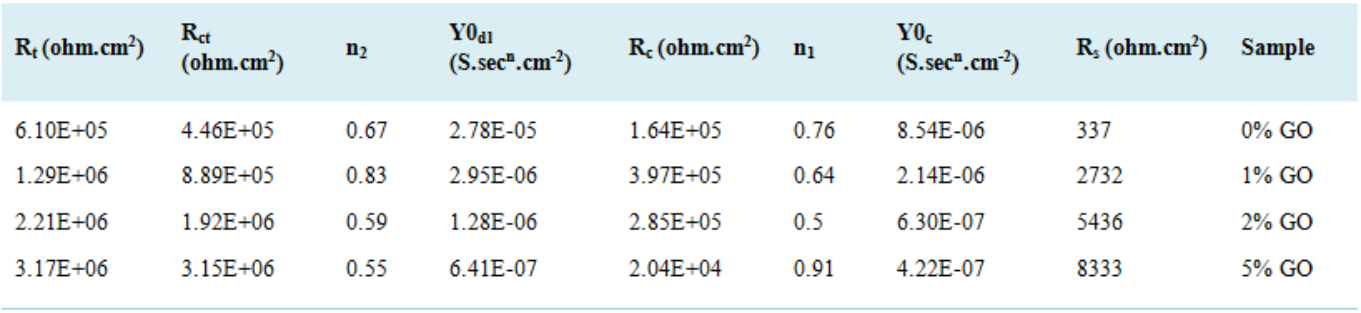
Rp (MOhm.cm2) | icorr (μA/cm2) | Ecorr vs SCE (V) | βc (V/dec) | βa (V/dec) | Sample |
|---|---|---|---|---|---|
0.225 | 0.175 | -0.102 | 0.179 | 0.184 | 0% GO |
0.669 | 0.112 | -0.052 | 0.314 | 0.384 | 1% GO |
1.573 | 0.061 | -0.026 | 0.473 | 0.415 | 2% GO |
1.704 | 0.036 | 1.34 | 0.169 | 0.863 | 5% GO |
2.1. How to Perform Electrochemical Tests
2.2. MTT Test
3.1. Evaluation of Coating Corrosion Resistance
3.2. Polarization Test Analysis
3.3. Assessment of Cytotoxicity Behavior by MTT-Assay
| [1] | J. B. Park, J. D. Bronzino, "Biomaterials, Principles and Applications", CRC Press, 2002, 21. |
| [2] | A. Rabeie, B. Thomas, B. Neville, J. W. Lee, J. W Coumo, " Microstructure, Mechanical Properties and Biological Responses to Functionally Graded HA Coatings", Materials Science and Engineering C, 27(2007) 523. |
| [3] | H. R. Bakhsheshi-Rad, Z. Hadisi, E. Hamzah, A. F. Ismail, M. Aziz, M. Kashefian, Drug delivery and cytocompatibility of ciprofloxacin loaded gelatin nanofibers-coated Mg alloy, Materials Letters, 2017. |
| [4] | Wang, X., Ding, B. and Li, B., “Biomimetic electrospun nanofibrous structures for tissue engineering,” Materials Today, Vol. 16, pp. 229–241, 2013. |
| [5] | Woodruff, M. A., and Hutmacher, W. H., “The return of a forgotten polymer : Polycaprolactone in the 21st century”, Progress in Polymer Science, Vol. 35, pp. 1217–1256, 2010. |
| [6] | Li M, Wang Y, Liu Q, Li Q, Cheng Y, Zheng Y, et al. In situ synthesis and biocompatibility of nano hydroxyapatite on pristine and chitosan functionalized graphene oxide. J Mater Chem B 2013; 1(4): 475–84. |
| [7] | Neelgund GM, Oki A, Luo Z. In-situ deposition of hydroxyapatite on graphene nanosheets. Mater Res Bull 2013; 48(2): 175–9. |
| [8] | Rodrı´guez-Lorenzo Luis M, Benito-Garzo´n Lorena, Barroso- Bujans Fabienne, Ferna´ndez Mar. Synthesis and biocompatibility of hydroxyapatite in a graphite oxide. |
| [9] | Ramakrishna, S., Fujihara, K. Teo, W. Yong, T. and Ramaseshan, R. “Electro spunnan of ibers : solving global issues,” Materials today, Vol. 9, pp. 40–50, 2006. |
| [10] | Hutmacher D. W. and Dalton P. D., Melt Electrospinning, Chem. Asian J., 6, 44-56, 2011. |
| [11] | Lee S. and Obendorf S. K., Developing Protective Textile Materials as Barriers to Liquid Penetration Using Melt-Electrospinning, J. Appl. Polym. Sci., 102, 3430-3437, 2006. |
| [12] | Ko J., Kan D., and Jun M. B., Combining Melt Electrospinning and particulated leaching for fabrication of porous microfibers, Manuf. Lett., 3, 5-8, 2015. |
| [13] | Karchin A., Simonovsky F. I., Ratner B. D., and Sanders J. E., Melt Electrospinning of Biodegradable Polyurethane Scaffolds, Acta Biomater., 7, 3277-3284, 2011. |
| [14] | Hochleitner G., Jungst T., Brown T. D., Hahn K., Moseke C., Jakob F., Dalton P. D., and Groll J., Additive Manufacturing of Scaffolds with Sub-Micron Filaments via Melt Electrospinning Writing, Biofabrication, 7, 35002, 2015. |
| [15] | Lyons J., Li C., and Ko F., Melt-Electrospinning Part I: Processing Parameters and Geometric Properties, Polymer, 45, 7597-7603, 2004. |
| [16] | Hochleitner G., Hummer J. F., Luxenhofer R., and Groll J., high definition fibrous poly(2-Ethyl-2-oxazoline) scaffolds through melt electrospinning writing, Polymer, 55, 5017-5023, 2014. |
| [17] | Dalton P. D., Grafahrend D., Klinkhammer K., Klee D., and Moller M., Electrospinning of Polymer Melts: Phenomenological Observations, Polymer, 48, 6823-6833, 2007. |
| [18] | Lyons J. and Ko F., Melt Electrospinning of Polymers: A Review, Polym. News, 30, 170-178, 2005. |
| [19] | Kong C. S., Jo K. J., Jo N. K., and Kim H. S., Effects of The Spin line temperature profile and melt index of poly on Melt-Electrospinning, Technical Report, 2009. |
| [20] | Reneker D., Kataphinana W., Theron A., Zussman E., nanofiber garlands of polycaprolactone by electrospinning, Polymer, 43, 6785-6794, 2002. |
| [21] | Li X., Liu H., Wang J., and Li C., Preparation and Characterization of polycaprolactone nonwoven mats via melt electrospinning, Polymer, 53, 248-253, 2012. |
| [22] | Ma M., Mao Y., Gupta M., Gleason K. K., and Rutledge G. C., Superhydrophobic Fabrics Produced by Electrospinning and Chemical Vapor Deposition, Macromolecules, 38, 9742-9748, 2005. |
| [23] | Muerza-Cascante M. L., Haylock D., Hutmacher D. W., and Dalton P. D., Melt Electrospinning and Its Technologization in Tissue Engineering, Tissue Eng., Part B: Rev., 21, 187-202, 2014. |
| [24] | Detta N. et al., Melt Electrospinning of Polycaprolactone and Its Blends with Poly (ethylene glycol), Polym. Int., 59, 1558- 1562, 2010. |
| [25] | Li X., Wang Z., Wang J., Liu J., and Li C., Preparation and properties of TPU micro/nanofibers by A layser melt-Elec trospinning System, Polym. Eng. Sci., 54, 1412-1417, 2014. |
| [26] | Lyons J. M., Melt-Electrospinning of Thermoplastic Polymers: An Experimental and Theoretical Analysis, Ph. D. Thesis, Drexel University, October 2004. |
| [27] | Bhardwaj N. and Kundu S. C., Electrospinning: A Fascinating Fiber Fabrication Technique, Biotech. Adv., 28, 325-347, 2010. |
| [28] | Li X., Zhang Y., Li H., Chen H., Ding Y., and Yang W., Effect of Oriented Fiber Membrane Fabricated via Needleless Melt electrospinning on water filtration efficiency Desalination, 344, 266-273, 2014. |
| [29] | Zhou H., Green T. B., and Joo Y. L., The Thermal Effects on Electrospinning of Polylactic Acid Melts, Polymer, 47, 7497-7505, 2006. |
| [30] | McCann J. T., Marquez M., and Xia Y., Melt Coaxial Electrospinning: A Versatile Method for The Encapsulation of Solid materials and fabrication of phase change nanofibers, Nano lett., 6, 2868-2872, 2006. |
| [31] | Dalton P. D., Joergensen N. T., Groll J., and Moeller M., Patterned Melt Electrospun Substrates for Tissue Engineering, Biomed. Mater., 3, 1-11, 2008. |
| [32] | Zong H., Xia X., Liang Y., Dai S., Alsaedi A., Hayat T., Kong F., and Hong Pan J., Designing Function-Oriented Artificial Nanomaterials and Membranes via Electrospinning and Electrospraying Techniques, Mater. Sci. Eng., C, 2017. |
| [33] | Li X., Liu H., Wang J., and Li C., Preparation and Characterization of PLLA/nHA Nonwoven Mats via Laser Melt Electrospinning, Mater. Lett., 73, 103-106, 2012. |
| [34] | Dutta P. K., Dutta J., and Tripathi V., Chitin and Chitosan: Chemistry, Properties and Applications, j. Sci. Ind. Res., 63, 20-31, 2004. |
| [35] | Sill T. J. and von Recum H. A., Electrospinning: Applications in Drug Delivery and Tissue Engineering, Biomaterials, 29, 1989-2006, 2008. |
| [36] | Ko J., Khadem Mohtaram N., Ahmed F., Montgomery A., Carlson M., Lee P. C. D., Willerth S. M., and Jun M. B. G., Fabrication of poly caprolactone microfiber scaffolds with Varying Topography and Mechanical Properties for Stem Cell-Based Tissue Engineering Applications, J. Biomater. Sci., Polym. Ed., 25, 1-17, 2014. |
| [37] | Cho D., Zhou H., Cho Y., Audus D., and Joo Y. L., Structural Properties and Superhydrophobicity of Electrospun Polypropylene Fibers from Solution and Melt, Polymer, 51, 6005- 6012, 2010. |
| [38] | Ma M., Hill R. M., Lowery J. L., Fridrikh S. V., and Rutledge G. C., Electrospun Poly(Styrene-Block-Dimethylsiloxane) Block Copolymer Fibers Exhibiting Superhydrophobicity, Langmuir, 21, 5549-5554, 2005. |
| [39] | singh., steely l., and ALLcock H. R., poly [bis(2,2,2-trifluo-roethoxy)phosphaz] superhydrophobic Nanofibers Langmuir, 21, 11604-11607, 2005. |
| [40] | Iqbal-Sabir M., Xu X., and Li L., A Review on Biodegradable Polymeric Materials for Bone Tissue Engineering Applications, J. Mater. Sci., 44, 5713-5724, 2009. |
APA Style
Fazlali, P., Tahernejad, M., Biglari, L., Eslami, M. (2024). Surface Modification of Ti-6Al-4V Alloy by Polycaprolactone-Graphene Oxide Composite Coating. International Journal of Materials Science and Applications, 13(2), 13-23. https://doi.org/10.11648/j.ijmsa.20241302.11
ACS Style
Fazlali, P.; Tahernejad, M.; Biglari, L.; Eslami, M. Surface Modification of Ti-6Al-4V Alloy by Polycaprolactone-Graphene Oxide Composite Coating. Int. J. Mater. Sci. Appl. 2024, 13(2), 13-23. doi: 10.11648/j.ijmsa.20241302.11
AMA Style
Fazlali P, Tahernejad M, Biglari L, Eslami M. Surface Modification of Ti-6Al-4V Alloy by Polycaprolactone-Graphene Oxide Composite Coating. Int J Mater Sci Appl. 2024;13(2):13-23. doi: 10.11648/j.ijmsa.20241302.11
@article{10.11648/j.ijmsa.20241302.11,
author = {Paria Fazlali and Mahrokh Tahernejad and Leila Biglari and Mahla Eslami},
title = {Surface Modification of Ti-6Al-4V Alloy by Polycaprolactone-Graphene Oxide Composite Coating
},
journal = {International Journal of Materials Science and Applications},
volume = {13},
number = {2},
pages = {13-23},
doi = {10.11648/j.ijmsa.20241302.11},
url = {https://doi.org/10.11648/j.ijmsa.20241302.11},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.ijmsa.20241302.11},
abstract = {In this research, Polycaprolactone-graphene oxide Nanocomposite coating was synthesized and characterized by Electrospinning on Ti6AL4V alloy. In order to create a uniform coating with optimal thickness, the effective parameters of Electrospinning coating, including solvent, polymer concentration, and bioceramic percentage, were investigated. Also, the cytotoxicity and corrosion tests were evaluated by the electrochemical polarization test method of the created coating in comparisons and different percentages. In order to characterize the coating, a test such as a scanning electron microscope was used. The results showed that as much as the amount of Graphene oxide is increased, the diameter of Nanofibers decreases. The diameter of Polycaprolactone Nanofibers was 1.3 micrometers, which increases to 56.0 micrometers by adding Graphene oxide. The results of the corrosion test showed that the use of Nano composite coating increased the corrosion resistance to the size of the coating. The nanocomposite coating consists of polycaprolactone nanofibers and graphene oxide, which mimics the behavior of the extracellular matrix and improves the biological and antibacterial behavior of the titanium surface. So far, there has been no report on the creation of this fibrous nanocomposite coating on titanium. The results of the cytotoxicity test showed that the use of Nanocomposite coating has effectively reduced the cytotoxicity on the scaffolds. By creating a polycaprolactone-graphene oxide nanofiber composite coating, the biological and antibacterial properties of titanium alloy will be improved and its corrosion resistance will probably change. In this project, the main question is extracting effective parameters in creating a composite coating on titanium surface by electrospinning method and characterizing and biological evaluation of the created coating.
},
year = {2024}
}
TY - JOUR T1 - Surface Modification of Ti-6Al-4V Alloy by Polycaprolactone-Graphene Oxide Composite Coating AU - Paria Fazlali AU - Mahrokh Tahernejad AU - Leila Biglari AU - Mahla Eslami Y1 - 2024/04/02 PY - 2024 N1 - https://doi.org/10.11648/j.ijmsa.20241302.11 DO - 10.11648/j.ijmsa.20241302.11 T2 - International Journal of Materials Science and Applications JF - International Journal of Materials Science and Applications JO - International Journal of Materials Science and Applications SP - 13 EP - 23 PB - Science Publishing Group SN - 2327-2643 UR - https://doi.org/10.11648/j.ijmsa.20241302.11 AB - In this research, Polycaprolactone-graphene oxide Nanocomposite coating was synthesized and characterized by Electrospinning on Ti6AL4V alloy. In order to create a uniform coating with optimal thickness, the effective parameters of Electrospinning coating, including solvent, polymer concentration, and bioceramic percentage, were investigated. Also, the cytotoxicity and corrosion tests were evaluated by the electrochemical polarization test method of the created coating in comparisons and different percentages. In order to characterize the coating, a test such as a scanning electron microscope was used. The results showed that as much as the amount of Graphene oxide is increased, the diameter of Nanofibers decreases. The diameter of Polycaprolactone Nanofibers was 1.3 micrometers, which increases to 56.0 micrometers by adding Graphene oxide. The results of the corrosion test showed that the use of Nano composite coating increased the corrosion resistance to the size of the coating. The nanocomposite coating consists of polycaprolactone nanofibers and graphene oxide, which mimics the behavior of the extracellular matrix and improves the biological and antibacterial behavior of the titanium surface. So far, there has been no report on the creation of this fibrous nanocomposite coating on titanium. The results of the cytotoxicity test showed that the use of Nanocomposite coating has effectively reduced the cytotoxicity on the scaffolds. By creating a polycaprolactone-graphene oxide nanofiber composite coating, the biological and antibacterial properties of titanium alloy will be improved and its corrosion resistance will probably change. In this project, the main question is extracting effective parameters in creating a composite coating on titanium surface by electrospinning method and characterizing and biological evaluation of the created coating. VL - 13 IS - 2 ER -